Abstract
Introduction
There is currently no standard of care for the upfront management of patients with mature T cell non-Hodgkin lymphomas (T-NHL). The role of anthracyclines in the treatment of T-NHL remains unclear and is also associated with significant toxicity. CEPP (cyclophosphamide, etoposide, procarbazine, and prednisone), a non-anthracycline regimen, is an effective salvage regimen for relapsed/refractory NHL and has shown a favourable toxicity profile. Since 2005, CEPP has been used in Singapore in the upfront treatment of T-NHL patients either with a contraindication to anthracyclines or at physicians' discretion. Our study aimed to assess the efficacy of CEPP in the upfront treatment of T-NHL.
Methods
A retrospective study of all patients with newly diagnosed T-NHL treated with CEPP with curative intent from 2005-2021 was undertaken across 2 national cancer centers in Singapore. Outcomes of this population was also compared with a 1:1 control group treated with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) in the same time period, and matched for age, T-NHL subtype, clinical stage and international prognostic index (IPI). Patient demographics, disease characteristics and clinical outcomes (progression free survival, PFS and overall survival, OS) were evaluated. None of the patients had upfront autologous transplantation.
Results
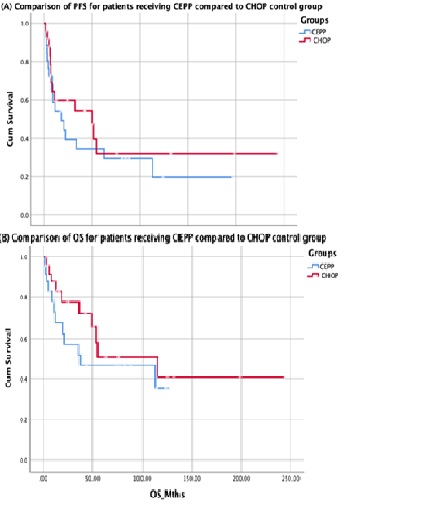
We identified 34 patients treated with CEPP who met the inclusion criteria. Clinical characteristics were as follows: Median age was 71 (range 39-85), 24 (71%) were male, 26 (76%) had advanced disease (Stage III-IV) and 21 (62%) had a high intermediate or high risk IPI ( IPI 3, 14 (41%) and IPI 4-5, 21 (21%)). The most common T-NHL subtypes were peripheral T-NHL (PTCL-NOS) not otherwise specified, 11 (32%) as well as angioimmunoblastic T cell lymphoma (AITL), 16 (47%). At a median follow up of 20 months (range 2-197months), the median PFS and OS were 17.6mths and 37.2mths respectively for the CEPP group. 15/34 (44%) CEPP patients have died (8 with lymphoma, 2 from treatment toxicity and 5 from unrelated causes). In the matched control comparison, the 5yr PFS and OS were both similar for patients treated with CEPP compared to patients in the CHOP control group, PFS 30% vs 32%, p= 0.43 and OS 47% vs 52%, p = 0.32, respectively (Figure 1)
Conclusion
Our findings show that CEPP is a well-tolerated regimen which can cure a proportion of patients with T-NHL even without autologous transplantation consolidation. Outcome of these patients do not seem to be significantly different compared to a similar population treated with standard CHOP. This study supports CEPP as a tolerable treatment option for selected patients who cannot tolerate anthracyclines and as an alternative to CHOP regimen for older patients who are not planned for ASCT.
Jeyasekharan: Turbine Ltd: Consultancy; Janssen: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Perkin Elmer: Other: travel funding ; MSD: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal